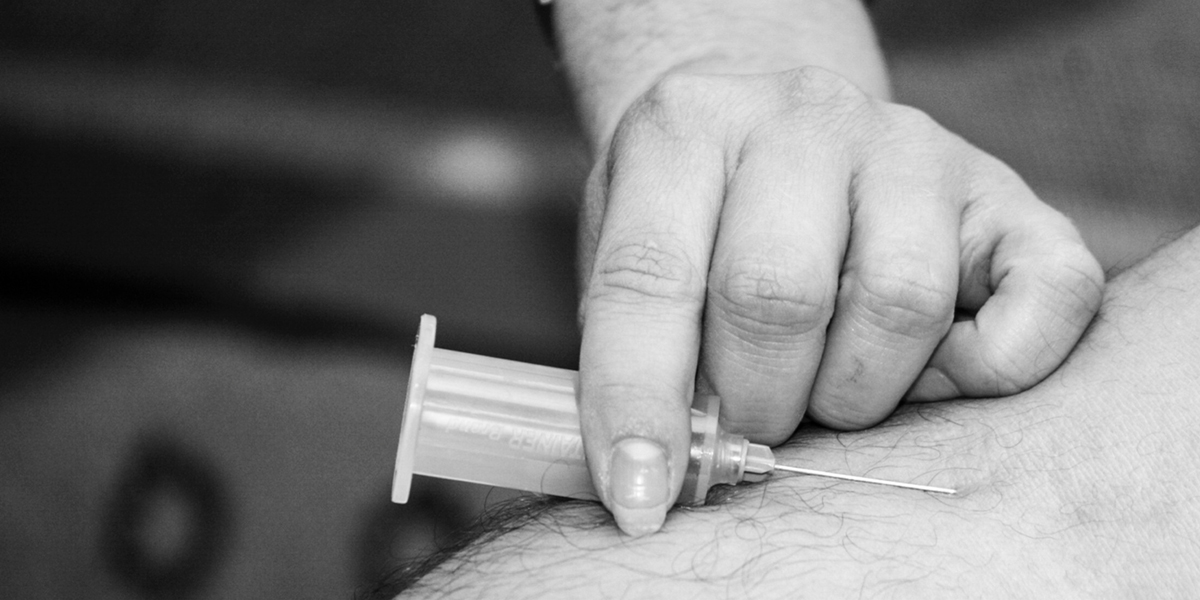
Sublocade, from Indivior Plc, is buprenorphine given by injection. The drug reduces the sensations of withdrawal symptoms in addiction patients. Administering the drug monthly could help patients comply to the treatment schedule, compared to other versions currently available such as daily oral doses in the form of pills or dissolving strips.
Effort to Reduce Stigmas Around Drugs
Approving the new drug treatment product is part of the Administration’s plan to reduce stigmas surrounding this class of medications. Buprenorphine is a synthetic opioid, and critics have expressed their opposition to medication assisted treatment (MAT). Instead, they favor changing users’ behavior to combat the current opioid crisis.
The FDA and other federal agencies are supporting MAT, which involves administering drugs and providing counseling to those affected. The White House referred to the situation as a “public health emergency” in October.
The FDA commissioner, Scott Gottlieb, released[1] a statement recently that said the FDA is “committed to expanding access to treatments that can help people pursue lives of sobriety.”
New Medication Available Early in 2018
According to reports[2], Sublocade will be made available to patients early in 2018, according to Indivior. The National Institute on Drug Abuse reports that in 2016, drug overdoses were responsible for taking the lives of more than 64,000 Americans. This figure includes overdoses caused by prescription drugs, such as fentanyl and OxyContin, and illicit ones like heroin. Clients who receive medication assisted treatment for drug addiction reduce their risk of death from all causes in half, according to the FDA, making this option a valuable one.
Mr Gottlieb stated that the FDA is currently working on guidelines that will get further treatment options for treating opioid addiction into the market more rapidly.
Sources:
[1] https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm587312.htm
[2] https://www.bloomberg.com/news/articles/2017-12-01/once-monthly-opioid-addiction-injection-cleared-for-u-s-sales